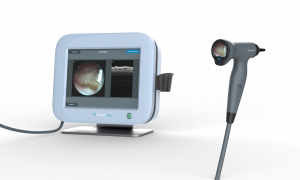
Congratulations to one of our portfolio companies, PhotoniCare, for achieving FDA Clearance! The company’s TOMi™ Scope has been granted a 510(k) clearance for its non-invasive imaging of the middle ear. The TOMi Scope helps to determine the presence or absence of fluid in the middle ear and to characterize the fluid type.
We are grateful for the steady leadership and diligent work by Ryan Shelton, Ryan Nolan, and the whole team to get to this point.